Research
Hybrid materials consist of inorganic and organic components. We mainly focus on semiconducting hybrid materials, which involve a combination of various metal halides with organic cations. They exhibit different coordination environments, such as octahedral or tetrahedral structures. The metals utilized are composed of both main group elements (e.g., Pb, Sn, In, Sb, Bi) and transition metals (e.g., Cu, Ag, Mn, Zn), while the halides are I, Br, and Cl. As for the organic cations, they can be linear or cyclic, aromatic or aliphatic, and either monoammonium or diammonium cations. The hybrid metal halides have different applications in energy conversion and optoelectronic devices such as solar cells, LEDs and detectors.
One of the rockstar hybrid materials is halide perovskites, which consist of six-coordinated octahedra connected by corner-sharing. They exhibit outstanding physical properties such strong absorption, long carrier lifetime and long diffusion length, which have led to their exceptional performances in optoelectronic devices. The major drawback of three-dimensional (3D) halide perovskites is their poor stability under moisture, heat and light. One of the solutions to this problem is to utilize lower-dimensional halide perovskite, which can be derived from 3D perovskites by slicing the octahedral layers in different directions and incorporating large organic cations called spacers. The hydrophobic organic cations create a protective “umbrella” layer, shielding the structure from environmental degradation.
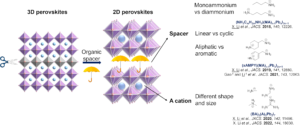
When the shape and size of the spacer cations meet specific requirements, two-dimensional (2D) perovskites can be synthesized. Otherwise, one-dimensional (1D) or zero-dimensional (0D) structures would form instead. When the coordination environment of the structure is different from octahedral, they are referred to as “hybrid metal halides” rather than “halide perovskites”. We also want to move away from the toxic Pb to other main group elements and transition metals to explore their optical, electronic and magnetic properties.